Some basic concepts and mole concepts
What is chemistry ?
- The branch of science that deals with the properties , composition and structure of elements and compounds.
- it is science of atoms , molecules and their transformation.
what is science ?
systematic knowledge of physical world and its phenomenon that involves observation and experimentation.
Properties of matter and their measurement.
\longrightarrow classified of matter and their measurements.
classified into unique chemical and physical properties :-
- Physical properties :- those properties which can be measuremd or observed without changing the identity or composition of the substance are known as physical properties . examples :- colour , M.P , B.P , odour etc.
- chemical properties :- those properties which describe a matter’s potential to undergo some chemical change are known as chemical properties . example:- acidity , basicity etc.
Measurement :- Any quantitative observation represented by a number followed by a unit in which it is measured is called measurement such as :- length , area , volume etc
Types of systems of measurements :-
- English systems
- Metric systems
\longrightarrow metric system is originated in france in 18th century is more convenient and widely used.
\longrightarrow came from metric systems
Types of units :-
- Base units
- derived units
1. Base units :- the physical quantity which is independent of any other other physical quantity.
\longrightarrow there are seven base units which is as follows with their symbol and SI units :-
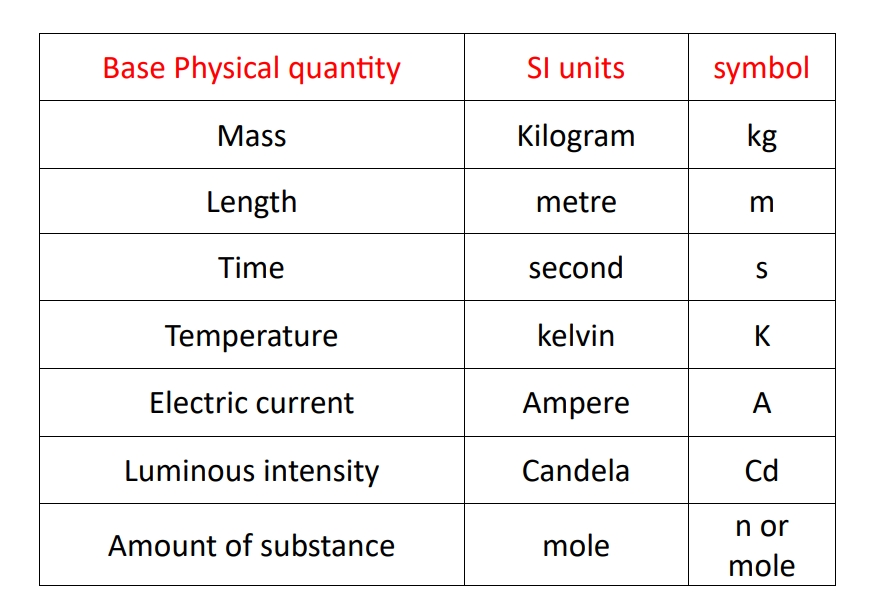
Derived units :- the units of all other physical quantities are derived out of those of the basic physical quantities the unit thus obtained are called derived units.
examples :- area , volume , density , velocity etc.
for more video lecture you can visit my YouTube or my website