All the model paper and previous paper are given below :-
Model paper list
model paper class 10
Following is the chapter of class 10 . and this section model paper class 10 will be chapter wise and common model paper
Chemical Reactions and Equations: Learn about the transformation of substances through chemical reactions, balancing equations, and understanding the types of reactions that occur in various chemical processes.
Acids, Bases, and Salts: Explore the properties of acids, bases, and salts, their reactions, and their importance in daily life, including their role in industries and agriculture.
Metals and Non-metals: Delve into the world of metals and non-metals, understanding their properties, extraction methods, and their applications in different fields.
Carbon and its Compounds: Explore the versatile nature of carbon, studying organic chemistry, hydrocarbons, and the various compounds formed by carbon, with a focus on their significance in our daily lives. visit here for lecture -1 , lecture – 2 , lecture – 3
Periodic Classification of Elements: Discover the organization of elements based on their properties in the periodic table, elucidating the trends, groups, and periods that govern their behavior.
Life Processes: Understand the fundamental processes of life, including nutrition, respiration, transportation, and excretion in living organisms, exploring the mechanisms that sustain life.
Control and Coordination: Uncover the intricacies of the nervous and endocrine systems, studying how organisms regulate and coordinate their physiological activities for optimal functioning.
How do Organisms Reproduce?: Explore the various modes of reproduction in plants and animals, understanding the processes of asexual and sexual reproduction, as well as the significance of reproduction in maintaining species.
Heredity and Evolution: Investigate the principles of heredity and evolution, studying the transmission of traits from one generation to the next and the mechanisms that drive evolutionary changes over time.
Light – Reflection and Refraction: Dive into the world of optics, examining the behavior of light when it interacts with surfaces, understanding the concepts of reflection and refraction, and exploring their applications in daily life.
Human Eye and Colourful World: Study the structure and functions of the human eye, along with the phenomena of dispersion, color formation, and their impact on our perception of the world around us.
Electricity: Explore the concepts of electric current, circuits, and electromagnetism, unraveling the principles that govern the flow of electricity and its applications in various devices.
Magnetic Effects of Electric Current: Understand the magnetic effects produced by electric currents, exploring the principles of electromagnetism and their applications in devices such as electric motors and generators.
Sources of Energy: Investigate different sources of energy, including conventional and renewable sources, assessing their advantages, disadvantages, and the need for sustainable energy solutions in the modern world.
Our Environment: Examine the components of the environment, environmental issues, and the importance of conservation, emphasizing the role of individuals in maintaining ecological balance.
Management of Natural Resources: Learn about sustainable practices in the management of natural resources, focusing on the responsible utilization and conservation of resources to ensure a balanced and healthy environment.

- Familiarize with exam pattern.
- Practice time management.
- Identify weak areas.
- Boost confidence for the actual exam.
Model paper 1 :-Chapter - 1(Chemical reaction and Equation)
Model paper 2 : previous year question paper class 10 Science
Time :- 2:30 hours
Max. marks :- 80
General instructions :-
Read the the the following instruction very carefully and follow them :-
- this question is consists of 39 questions . All questions are compulsory.
- Question paper is divided into FIVE section – section A , B , C, D , and E.
- In section A question number from 1 to 20 are multiple choice question(MCQ) carrying 1 mark each.
- In section B question number 21 to 26 are very short answer type question carrying 2 marks each. Answer to these question should be in the range of 30 to 50 words.
- In section C question number 27 to 33 are short answer type question carrying 3 marks each . Answer to these question should be in the range of 50 to 80 words.
- In section D question number 34 to 36 are long type questions carrying 5 marks each. Answer to these question should be in the range of 80 to 120 words.
- In section E question number 37 to 39 are of 3 sources based /case based unit assessment carrying 4 marks each with sub-parts.
- There is no overall choice / however , an internal choice has been provided in some sections.
SECTION - A
Select and write one most appropriate option out of the four options given for each of the questions 1-20
1. Metal oxide generally react with acids , but few oxides of metal also react with bases. such metallic oxides are 1
I). MgO
III). Al_2 O_3
II). ZnO
IV). CaO
(a). I and II
(b). II and III
(c). III and IV
(d). I and IV
2. Few drops of aqueous solution of ammonium chloride are put on a universal indicator paper. the paper turns pink. study the table and choose the correct option. 1

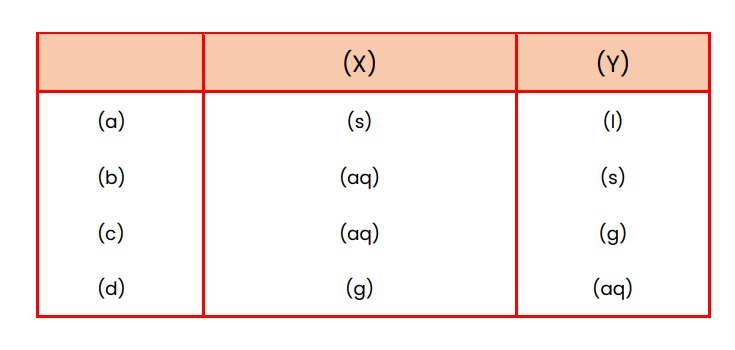
3. Select the appropriate state symbols of the products given as X and Y in the following chemical equation by choosing the correct option from table given below. Zn(s) + H_2 SO_4 (l) \rightarrow ZnSO_4 (X) + H_2 (Y)
4. Two salts X and Y are dissolve in water separately. when phenolphthalein is added to these two solutions the solution X turns pink and the solution Y does not show any change in colour , therefore X and Y are :-
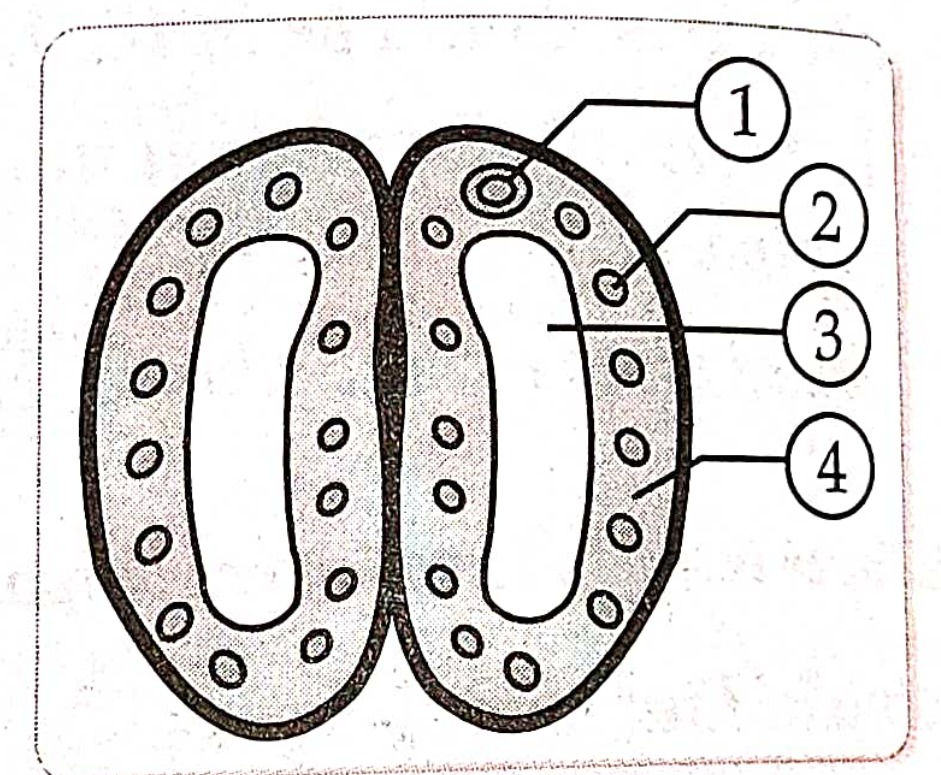
5. In the given diagram of a closed stomata : (1) , (2) , (3), and (4) respectively are
(a). nucleus , chloroplast , guard cell , vacuole
(b). nucleus , chloroplast , vacuole , guard cell
(c). chloroplast , nucleus , vacuole , guard cell
(d). vacuole , guard cell , nucleus , chloroplast.
6. Walking in a straight line and riding a bicycle are the activities which are possible due to the part of the brain. choose the correct location and name of this part from the given table:

7. A student wants to obtain an erect image of an object using a concave mirror of 10 cm focal lenght . What be the distance of the object from mirror ?
(a). Less than 10 cm
(b). 10 cm
(c). between 10 cm and 20 cm
(d). more than 20 cm
8. Bronze is an alloy of :
(a). Copper and Zinc
(b). Aluminium and Tin
(c). Copper, Tin, Zinc
(d). Copper and Tin
9. In an experiment with pea plants, a pure tall plant(TT) is crossed with a pure short plants (tt). The ratio of pure tall plant to pure shorts plants in F_2 generation will be :
(a). 1:3
(c). 1:1
(b). 3:1
(d). 2:1
10. Study the given figure of a food web and identify the primary consumer in the food web :
(a). Mice and Bear
(b).Rabbit and Cat
(c).Rabbit and Fox
(d).Mice and Rabbit

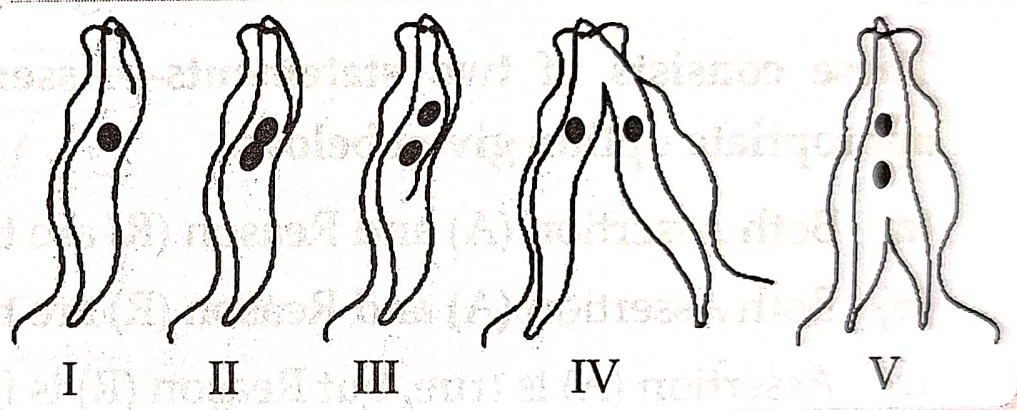
11. Choose the correct order of the stages of binary fission in Leishmania.
(a). I, II, III IV, V
(b).I, III, II, V, IV
(c). I, III, V, II, IV
(d). I, II, III, V, IV
12. Consider the following chemical equation I and II.
I. Mg + 2HCl \rightarrow MgCl_2 + H_2
II. NaOH + HCl \rightarrow NaCl + H_2 O
The correct statement about these equation I and II
(a) ‘I’ is a displacement reaction and ‘II’ is a decomposition reaction.
(b). ‘i’ is a displacement reaction and ‘II’ is double displacement reaction.
(c) Both ‘I’ and ‘II’ are displacement reaction.
(d) Both ‘I’ and ‘II’ are double-displacement reaction.
13. In the following diagram showing dispersion of white light by a glass prism, the colours ‘p’ and ‘Q’ respectively are –
(a) Red and Violet
(b) Violet and Red
(c) Red and Blue
(d) Orange and Green

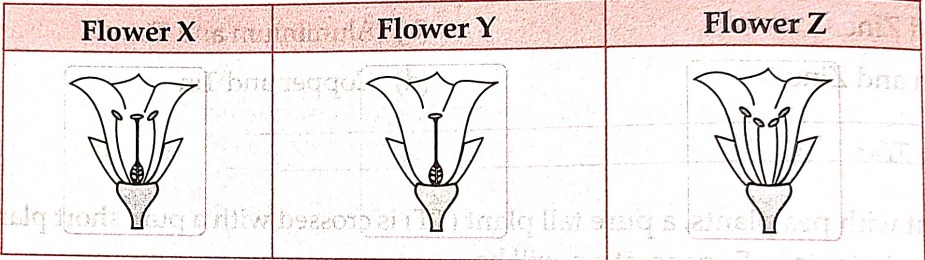
14.Consider the following three flower namely X, Y, Z. Which flower (s) would develop into a fruit?
(b). ‘Z’ ONLY
(C). ‘X’ and ‘Y’ only
(d). ‘Y’ and ‘Z’
15. The magnetic field inside a long straight current carrying solenoid :
(a). Is zero
(c).Increases as we move towards its end.
(b). Decreases as we move towards its end.
(d).Is same at all points
16. In human eyes the part which allows light to enter into the eye is:
(a). Retina
(c).Eye lens
(b).Pupil
(d).Cornea
These consists of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below :
(a). Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation(A).
(b). Both Assertion (A) and Reason (R) are true Reason (R) IS Correct explanation (A).
(c) Assertion (A) is true, but Reason (R) is false.
(d) Assertion (A) is false, but Reason (R) is true.
17. Assertion (A): It is advised that while diluting an acid one should add water to acid and not acid to water keeping the solution continuously stirred.
Reason (R): The process of dissolving an acid into water is highly exothermic.
18. Assertion (A): The energy which passes to the herbivores does not come back to autotrophs.
Reason (R): The flow of energy in a food chain is unidirectional.
19.Assertion (A): Amoeba takes in food using finger like extensions of cell surface.
Reason (R): In all unicellular organisms, the food is taken in by the entire cell surface
20.Assertion (A): Melting point and boiling point of ethanol are lower than that of sodium chloride.
Reason(R): The forces of attraction between the molecules of ionic compounds are very strong.
SECTION B
Q. No 21 to 26 are very short Answer Questions.
21. State whether the given chemical reaction is redox reaction or not . justify your answer .
MnO_2 + 4HCl \rightarrow MnCl_2 + 2H_2 O + Cl_222. (a). List two difference between the movement of leaves of a sensitive plant and the movement of shoot towards light.
or ,
(b) what happens at synapse between two neurons ? state briefly .
23. Given the name of the enzyme presents in the fluid in our mouth cavity :
State the gland which produce it. What would produces it. What would happen to the gland stops secreting this enzymes.
24.Let the resistance of an electrical device remain constant, while the potential difference across its two ends decreases to one fourth of its initial value. What change will occur in the current through it? state the law which helps us in solving the solving the above stated question.
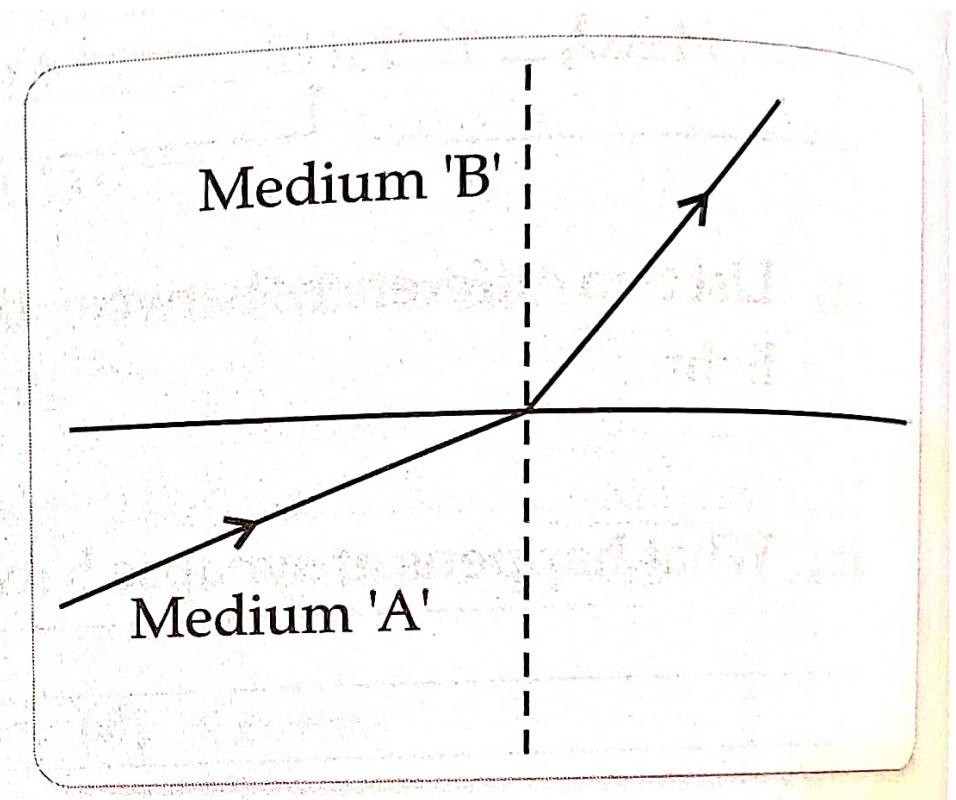
25. A light rays enters from medium A to medium B as shown in the figure.
(a) which one of the two media is denser w.r.t other medium? justify your answer.
(b) if the speed of light in medium A is V_A and B is V_B what is the refractive index of B with respect to A ?
OR
(a)A ray of light skirting from diamond is incident on the interface separating diamond and water . draw a labeled ray diagram to show the refraction of light in this case.
(b) Absolute refractive indices of diamond and water are 2.42 and 1.33 respectively . find the value of refractive index of water w.r.t diamond.
26. State the rule to determine the direction of (a) magnetic field produced around a straight conductor carrying current and (b) force experienced by a current carrying straight conductor placed in a magnetic field which is perpendicular to it .
SECTION C
Q.No 27 to 33 are short answer question.
27. Explain the process of transport of oxygenated and deoxygenated blood in a human body.
28. (a) A substance X is used as a building materials and is insoluble in water when it reacts with dil. HCl , it produces a gas which turns lime water milky.
(i) write the chemical name and formula of ‘X’
(ii) Write chemical equation for the chemical reactions involved in the above statements.
OR,
(b) A metal ‘M’ on reacting with dilute acid liberates a gas ‘G’ . The same metal also liberate also gas ‘G’ when reacts with base .
(i) wtite the name of gas ‘G’.
(ii) how will you test the presence of this gas ?
(iii) write chemical equations for the reaction of the metal with (1) and acid and (2) a base.
29. (a) Name the gland and the hormone secreted by it in scary situations in human beings list any two responses shown by our body when this hormone into the blood
OR
(b). In the given diagram
(i) Name the parts labelled A, B, C,
(ii) write the functions of A and C.
(iii) Reflex arcs have evolved in animals ? Why?
30. With the help of an appropriate example, justify that some of the chemical reactions are determined by
(a) Change in temperature
(b) Evolution of gas, and
(c) Change in color
Given chemical equation for the reaction involved in each case.
31. State reasons for myopia. With the help of ray diagrams, show the
(a) image formation by a myopic eye, and
(b) correction of myopia using an appropriate lens.
32. What is a solenoid ? When does a solenoid behave as a magnet? Draw the pattern of the magnetic field produced inside it showing the directions of the magnetic field lines.
33. (a) Writes the percentage of (i) solar energy capture by the autotrophs and (ii) energy transferred from autotrophs to the next level in a food chain.
(b) What are trophic levels? Why do different food chains in an ecosystem not have more than four to five trophic levels? Give reason.
SECTION D
Q. No.34 to 36 are Long Answer Questions.
34 . (a) (i). A compound “A” with a molecular formula of C_2 H_4 O_2 reacts with a base to give salt and water . identify ‘A’ , state it nature and the name of the functional group it possesses. write chemical equation for the reaction involved.
(ii). when the above stated compound ‘A’ reacts with another compound ‘B’ having molecular formula C_2 H_6 O in the presence of an acid , a sweet smelling compound ‘C’ is formed .
- identify ‘B’ and ‘C’.
- State the role of an acid in this reaction.
- write chemical equation for the reaction involved .
OR ,
(b) (i). Name the compound formed when ethanol is heated at 443 K in the presence of conc. H_2 SO_4 and draw its electron dot structure . state the role of conc. H_2 SO_4 in this reaction.
(ii) what is hydrogenation ? Explain it with the help of a chemical equation. state the role of this reaction in industry.
35. Given reason for the following:
(a) During reproduction inheritance of different proteins will lead to altered body designs.
(b) Fertilization cannot take place in flowers if pollination does not occur.
(c) All multicellular organisms cannot give rise to new individuals through fragmentation or regeneration.
(d) vegetative propagation is practiced for growing only type of plants.
(e) The parents and off-springs of organisms reproducing sexually have the same number of chromosomes.
36. (a) (i) What is meant by resistance of a conductor? Define its SI unit.
(ii) List two factors on which the resistance of a rectangular conductor depends.
(iii) How will the resistance of a wire be affected if its.
(1) length is doubled, and
(2) radius is also doubled?
Give justification for your answer.
(b)In an electric circuit three bulls of 100W each are connected in series to a source. In another circuit set of three bulbs of the same wattage are connected in parallel to the same sources.
(i) Will the bulb in the two circuits glow with same brightness ? Justify your answer.
(ii) Now, let one bulb in both the circuits get fused. Will the rest of the bulbs continue to glow in each circuit ?
Give reason for your answer.
SECTION E
Q . No 37 to 39 are case based /data based question with 2 and 3 short sub-parts . internal choice is provided in one of these sub-parts .
37. On the basis of reactivity metals are grouped into three categories :
(i). Metals of low reactivity
(ii). Metals of medium reactivity
(iii) Metals of high reactivity
Therefore metals are extracted in pure form from their ores on the basis of their chemical properties .
Metals of high reactivity are extracted from their ores by electrolysis of the molten ore.
Metals of low reactivity are extracted from their sulphide ores , which are converted into their oxides .The oxides of these metals are reduced to metals by simple heating .
(a). Name the process of reduction used for a metals that gives vigorous reaction with air and water both.
(b) Carbon cannot be used as a reducing agent to obtain aluminium from its oxide ? why?
(c)Describe briefly the method to obtain mercury from cinnabar . write the chemical equation for the reactions involved in the process.
OR
(c)Difference between roasting and calcination giving chemical equation for each.
38. All human chromosomes are not paired . Most human chromosomes have a maternal and paternal copy and we 22 such pairs. But one pair sex chromosomes is odd in not always being a perfect pair . woman have a perfect pair of sex chromosomes . but man have a mismatched pair in which one is normal sized while the other is short one.
(a). In human , how many chromosomes are present in a zygote and in each gamete .
(b) A few reptiles rely entirely on environmental cues for sex determinations comment.
(c) The sex of a child is matter of chance and none of the parents are considered to be responsible for it .justify it through flow chart only.
OR,
(c) why do all gametes formed in the human females have an X-chromosomes ?
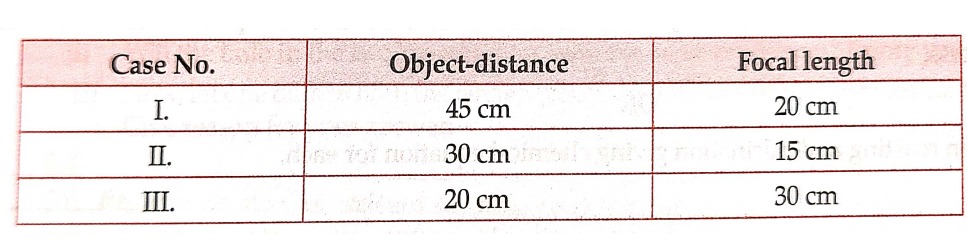
39.Student took three concave mirrors of different focal lengths and formed the experiment to see image formation by placing an object different distances with these mirrors as shown in the following table.
Now , answer the following questions :-
(a) List two properties of image formed in case I
(b)In which one the cases given in the table the mirror will form real image of same size and why ?
(c)Name the type of mirror used by dentists . Give reason why do they use such type of mirrors
OR,
(c) Look at the table and the situation (object distance and focal length ) which resembles the situation in which concave mirrors are used as shaving mirrors? Draw a ray diagram to show the image formation in this case.