Index
Chemical Reactions :-
Chemical reactions are fundamental processes in chemistry where substances, called reactants, undergo transformation into new substances, known as products. we can also say that when more than two substance reacts with each other and form new substance. These reactions involve the breaking and forming of chemical bonds between atoms, resulting in the rearrangement of atoms to create different compounds. in the chemical reaction following changes takes place
- Nature of substances changes
- phase of substance changes
- properties of the substances changes.
Examples of the chemical reaction :-
reaction equation – (1)
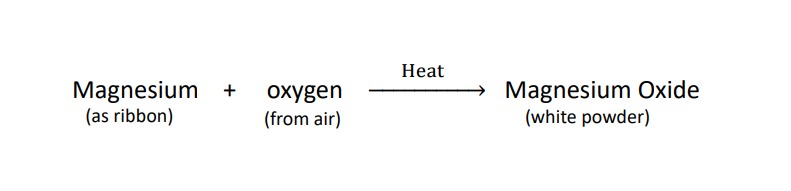
Burning of magnesium in air to form magnesium oxide is an example of a chemical reaction.
During the chemical reaction atoms of one elements do not change into those of another elements , only a rearrangement of atoms take place in a chemical reaction.in the chemical reaction it have two important part which is
- Reactant
- product
Reactant :-
the substance which take part in a chemical reaction are called reactant. it is the substance present before the reaction.it is generally written in the left side of the equation. all the reactants interact with each other then the reactions proceed.
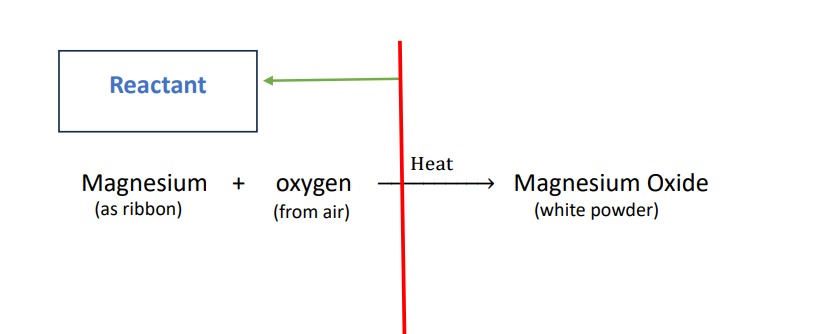
Here the reactant are Magnesium and oxygen.
Product :-
the new substance produced as a result of chemical reaction are called product. it is written as in right side of the chemical equation.it has new property ,totally different from the property of reactant.
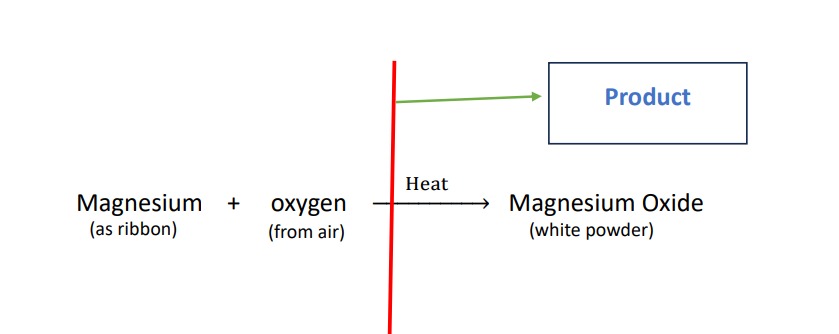
Here the product is Magnesium oxide which is white powder
in our daily life there are large number of reaction occurring like formation of curd , cooking of food , digestion of food in our body. process of respiration , fermentation of grapes , Rusting of iron , Burning of fuel , burning of candle wax , ripening of fruits etc all these are example of chemical reactions.
Characteristics of chemical reactions :-
the conversation of reactant into product in a chemical reaction is accompanied by some features which can be observed easily , following is the important characteristics of chemical reactions :-
- Evaluation of gas
- formation of a precipitate
- change of color
- change in temperature
- change in state.
Evolution of gas :-
some chemical reactions are characterized by the evolution of gas . example :- when a zinc reacts with Sulphuric acid the bubble of hydrogen gas are produced.
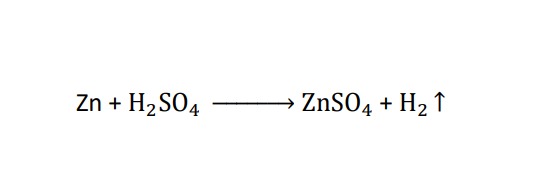

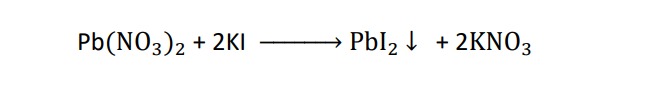
Formation of precipitate :-
some reactions are characterized by formation of a precipitate. when a potassium iodide solution is added to a solution of lead nitrate , then yellow precipitate of lead iodide is formed.

change in color :-
some chemical reactions are characterized by the change in color. when sulphur dioxide reacts with acidified potassium dichromate solution is characterized by change in color from orange to green.

change in temperature :-
some chemical reactions are characterized by change in temperature. example :- when quicklime(also known as ‘choona’) reacts with water then slaked lime is formed and lots of heat is produced.
Change in state :-
some chemical reactions are characterized by the change in state :- when wax(mombatti) is burned then water and carbon dioxide are formed where solid is converted into liquid and gas.
Types of chemical reactions :-
following is the types of chemical reactions :-
Combination reactions :-
those reactions in which two or more substance combine to form a single substance are called combination reaction. in this reaction , two or more elements can combine to form a compound , and two or more compounds can combine to form a new compounds.
examples of combination reactions are :-

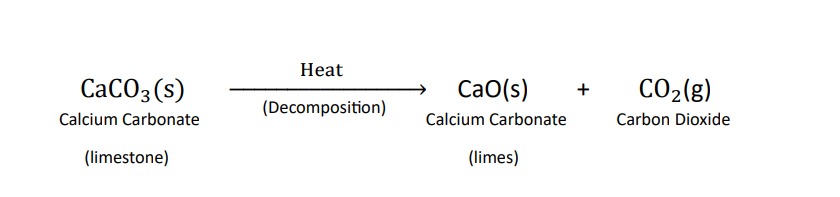
Decomposition reactions :-
Those reactions in which a compound splits up into two or more simpler substance are known as decomposition reactions .this reaction is carried out by applying heat , light or electricity. heat , light or electricity provide energy which breaks the bond between compounds and then compounds break into simpler compounds
examples of decomposition reactions :-
Displacement reaction :-
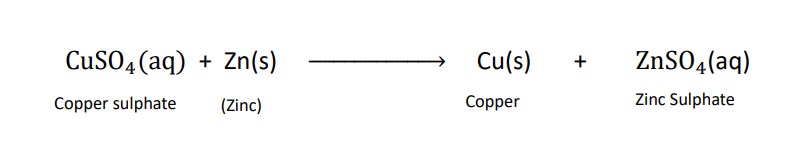
those reactions in which one elements takes the place of another element in a compound are known as displacement reaction.
examples of displacement reactions :-
Double Displacement reactions :-
those reaction in which two compounds reacts by an exchange of ions to form two new compounds are called double displacement reactions. this type of reaction usually occurs in solution and one of the products being insoluble , precipitate out.
examples of double displacement reactions :-

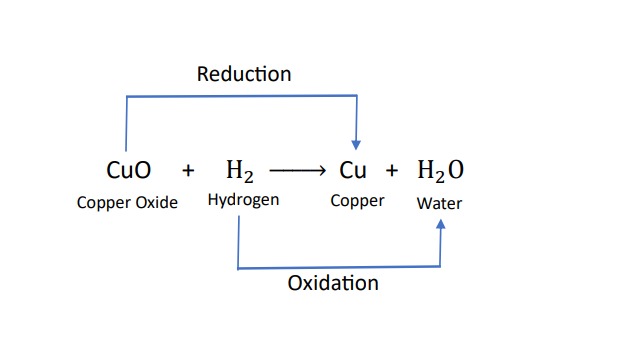
Redox reaction :-
The reaction in which oxidation and reduction at the same time in a single reaction.
Oxidation :-
the addition of oxygen to a substance is called oxidation .
The removal of hydrogen from a substance is also called oxidation
Reduction :-
the addition of hydrogen to a substance is called reduction
the removal of oxygen from the a substance is also called reduction.
Examples of Redox reaction :-
If You want to visit video lecture of Redox reaction and other Chemistry video please click the below link.