Table of Contents
Conservation of mass :-
we have learned about reactant and product in the chemical reaction section. now we will learn the conservation of mass and at the below of the theory there is video lecture you can go through that.
⇒ stated by Lavoisier in 1774.
⇒ matter can neither be created nor destroyed in the chemical reactions . it means whatever mass is reacting in the reaction all amount are known and it does not vanish after the reaction it means we used to get the new product from the initial chemicals. by this law we can say that mass will be converted into one form to another form but it does not destroy.
★ example :- we are cooking food , the mass of total raw materials used before the cooking is same as the total mass of cook food.
⇒ Number of various type of atoms in the reactant must be equal to the number of same type of atoms in the product.
⇒ in all chemical change the total mass if system remains constant.
⇒ This law is tested by landolt.
Following is the two possibility about reactions :-
- if Reaction is complete
- if reaction is incomplete
1. If reaction is complete :-
in this case all the reactant will be converted into the product. then we can say that :-
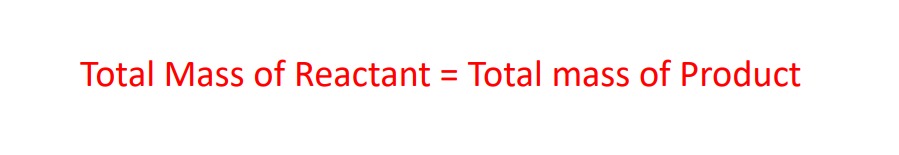
now let’s understood this with a hypothetical reaction example :-
A + B + C → D + E + F
where ,
- mass of A = ma
- mass of B = mb
- mass of C = mc
- mass of D = md
- mass of E = me
- mass of F = mf
A + B + C → D + E + F
from the above reaction :-
at t = 0(when reaction start) : all the reactant will be present then , total mass of reactant = ma + mb + mc
and mass of product at t = 0 is 0
and then,
at time t = t1 : all the reactant will convert into product then the mass of reactant is 0 and
mass of product at t = t1 , total mass of product = md + me + mf
then we can write from the above theory that
ma + mb + mc = md + me + mf
2. If reaction is incomplete :-
in this case all the reactant will not be converted into the product. then we can say that :-

now let’s understood this also with a hypothetical reaction example :-
A + B + C → D + E + F
where ,
- mass of A = ma
- mass of B = mb
- mass of C = mc
- mass of D = md
- mass of E = me
- mass of F = mf
- mass of unreacted A = m^'_a
- mass of unreacted B = m^'_b
- mass of unreacted C = m^'_c
A + B + C → D + E + F
from the above reaction :-
at t = 0(when reaction start) : some reactant will convert into product and some will be present unreacted then , total mass of reactant = ma + mb + mc
and mass of product at t = 0 is 0
and then,
at time t = t1 : some reactant will convert into product and some remains in the container then the mass of unreacted reactant is = m^'_a + m^'_b + m^'_c and
mass of product at t = t1 , total mass of product = md + me + mf
then from above theory
total mass of reactant = total mass of product + mass of unreacted reactant
ma + mb + mc = (md + me + mf) + ( m^'_a + m^'_b + m^'_c )
Balancing of chemical equations
when you hear balancing of anything weighing scale comes in mind. we put equal mass on both side of weighing scale whether we are measuring any object or any chemical. In this section we will study about balancing of chemicals which is used in the reaction because we know that conservation of mass is applied everywhere in the chemical reactions .we will discuss 3 method of balancing the chemical equation with examples.
Method - 1 :- Common procedure
In this method we will balance each atoms one by one and follow the steps to complete the balancing of chemical equations.
there is some rule you can follow to balance the chemical equations :-
- First balance atoms other than O and H atoms.
- then balance first H and then balance O atoms
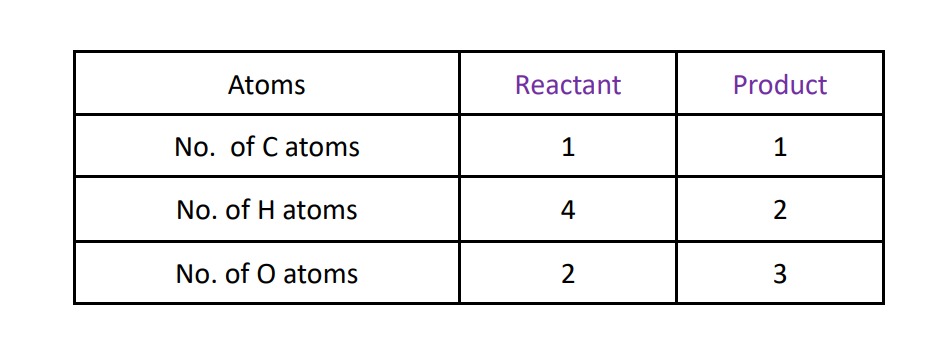
reaction of oxidation of methane :-
CH4 + O2 ⟶ CO2 + H2OStep – I:- write number of atoms of all the atoms
Step – II :- Balance the H atom by multiplying with 2 in the product side then the reaction will be :-
CH4 + O2 ⟶ CO2 + 2H2O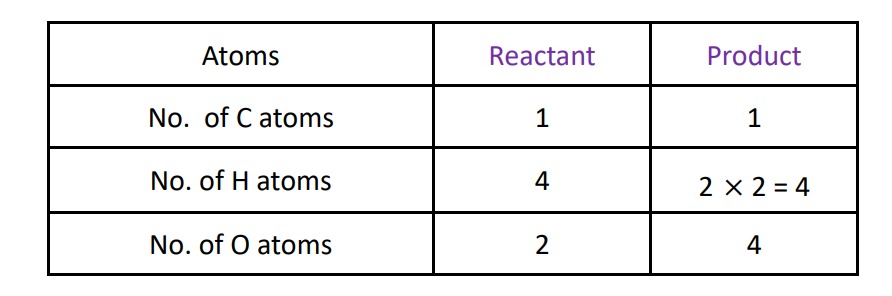
Step – III :- Balance the O atom by multiplying with 2 in the reactant side then the reaction will be :-
CH4 + 2O2 ⟶ CO2 + 2H2O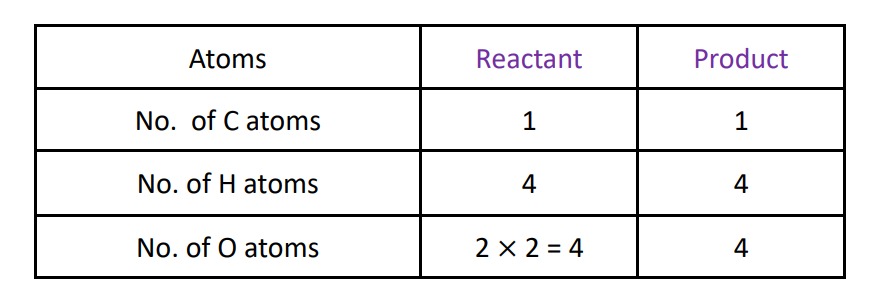
Now , look the number of various types of atoms in the reactant and product side , we have all atoms are in equal number in reactant and product side. hence we can say it is balance equation .
Method - 2 : Hit and trial
In this method , randomly we will put the numerical value of coefficient of reaction until we don’t get the correct value so that reaction would balanced. sometime this method is easy and don’t take time to balance the chemical equation but sometime it takes too much time in guessing the appropriate number to balance the equation. examples :-
CH4 + O2 ⟶ CO2 + H2Ofirst we will balance C atom. :- in the above reactions number of C atoms is same in both side
now , we will balance the H atom by multiply with 2 in the H2O then ,
CH4 + O2 ⟶ CO2 + 2H2Owe will balance the O atom by multiplying with 2 in the O2 then our reaction will be balanced
CH4 + 2O2 ⟶ CO2 + 2H2OMethod - 3 : algebraic expression
In this method we will the chemical equation by solving algebraic equation in different variable. we will balance the equation step by step . let’s take the one chemical equation :-
CH4 + O2 ⟶ CO2 + H2OStep – I :- Write variable like a , b , c and d as the balanced coefficient of the given equation.
aCH4 + bO2 ⟶ cCO2 + dH2Ostep – II :- from the equation in the step – I , we will solve the algebraic expression to find the value of a , b , c and d .
for C atoms
a = c …..(i)
For O atoms
2b = 2c + d .. (ii)
for H atoms
4a = 2d ….(iii)
then find the value of b , c and c in terms of a
2b = 2a + 2a
2b = 4a
b = 2a …(iv)
put the value of equation (i) , (iii) and (iv)
aCH4 + 2aO2 ⟶ aCO2 + 2aH2Ohence balanced equation is :-
CH4 + 2O2 ⟶ CO2 + 2H2Oyou may interested in the following :-